We want to simplify endoscopy
The single most important question in healthcare today is how to improve patient outcomes with the resources available. And as the world population increases and life expectancy expands, the pressure mounts on hospital budgets, workflow efficiency and, ultimately, patient safety.
Flexible endoscopes raise specific problems because they are costly to purchase, reprocess and repair, they are not always available when you need them, and their use risks exposing patients to infections.
We believe that the challenges in flexible endoscopy should be addressed with single-use devices, and for more than a decade we have been harnessing the technology.
Single-use endoscopy is the future
Increasing concern of cross-contamination is led by outbreaks
There is a need for a change in endoscopy
Mar 2015
FDA guidance on reprocessing
FDA issues final guidance on reprocessing of medical devices
Aug 2015
FDA WARNING LETTERS ISSUED
FDA issues warning letters to Olympus, Pentax and Fujifilm for failing to report MDRs to FDA on reusable scopes
Sep 2015
FDA WARNING STATEMENT
FDA issues safety communication regarding infections caused by reprocessing process on flexible bronchoscopes
Nov 2015
IMPROPER CLEANING SPREADING DISEASES
Inadequate “Cleaning of Flexible Endoscopes Before Disinfection Can Spread Deadly Pathogens” is #1 on ECRI Top 10 Health Technology Hazards for 2016
Jan 2017
CDC SAFETY ALERT ISSUE
CDC releases “Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the HICPAC
Mar 2018
FDA WARNING LETTERS ISSUED
FDA warns Olympus, Pentax and Fujifilm to prevent infections associated with the transmission of bacteria from contaminated duodenoscopes
Reusable endoscopy
A complex and costly setup putting patients at risk
Single-use endoscopy
A simple and cost-effective setup eliminating infection risks
In 2009, Ambu launched the world’s first single-use flexible endoscope
By 2020, Ambu will provide single-use across all flexible endoscopy areas
Ambu will offer scopes within all flexible endoscopy areas including sterile, single-use HD duodenoscopes, colonoscopes and gastroscopes
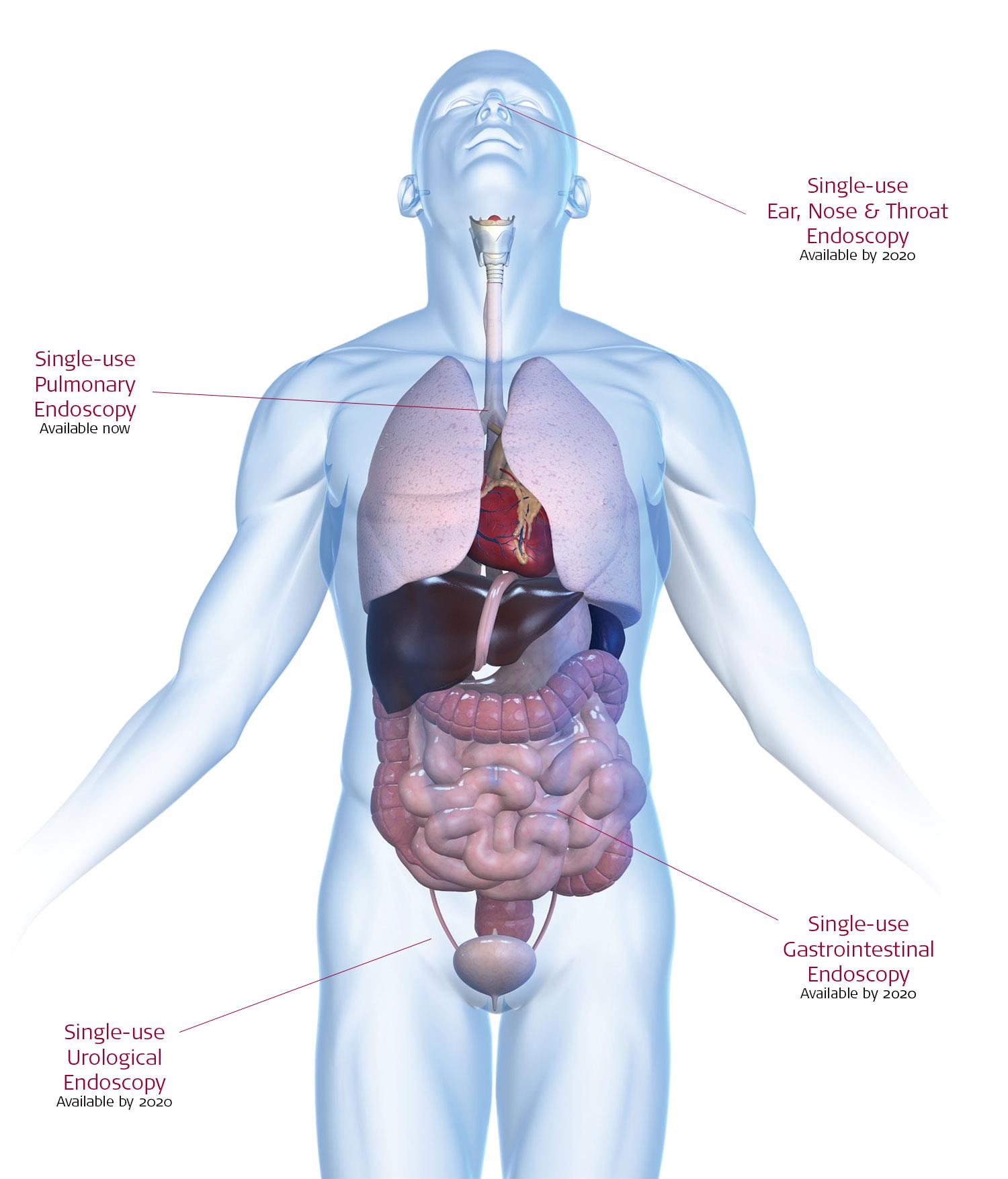
Single-use bronchosopes
Read more about Ambu's single-use, high quality bronchoscope; the Ambu® aScope™ 4 Broncho, which comes in three sizes.
Our latest proof of ideas that work for life.
The convenience and the safety of having the scopes right here has been a game changer for us.
The two main advantages are: it is so quick, efficient and available that it allows us – in the patient who has a suspected pulmonary infection – to get in and get the appropriate microbiological sampling – often before the time the first dose of antibiotic is administered.
The second advantage, which is probably equally important, is it’s a disposable scope. In the ICU, we’re dealing with resistant organisms, and reusable scopes can be colonized. They can have their channels contaminated. With single-use scopes, you use it and throw it away. And you have a sterile new scope for the next patient. It’s great from an infection prevention point of view, as far as we’re concerned, as well.
Eric C. Feucht, MD
Critical Care Medicine and Medical Director of Respiratory Therapy
at Metro Health Hospital in Ann Arbor
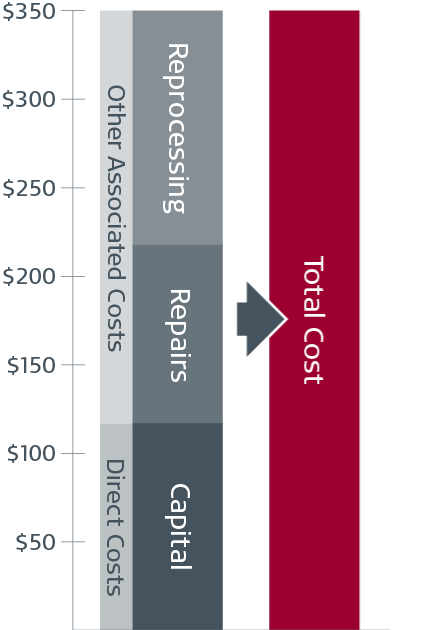
Comprehensive reprocessing is complex, time-consuming, and costly 1,2,3,4,5,6,7
$350
Average cost burden for reusable scopes
$300
Cost of Ambu aScope for every procedure
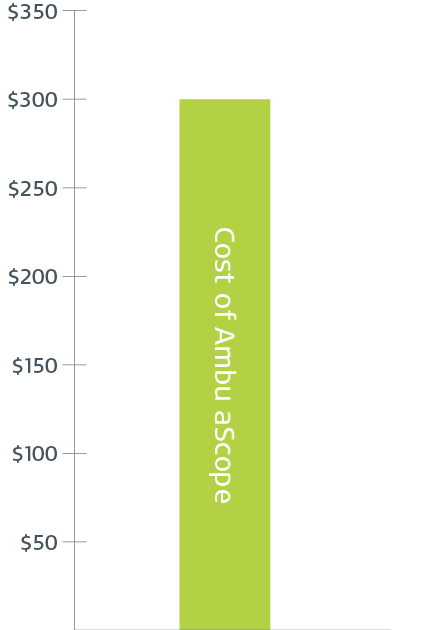
Steps in reprocessing
The basic design of the reprocessed endoscope has remained virtually unchanged for over half a century since the advent of the first flexible endoscope in the 1960s. The endoscopist, and device manufacturers, have been
limited by the physical constraints of the endoscope working channel. A disposable platform can change this by enabling the development of endoscopes tailored to the requirements of the accessory. This can open new frontiers of endoscopic intervention.
Kenneth F. Binmoeller, MD, FACG, FASGEDirector
Interventional Endoscopy Services California Pacific Medical Center
I believe disposable endoscopy will play a very important role in gastroenterology. Patients are understandably concerned about recent reports of infection transmission. We need to explore the possibility of using disposable devices in GI endoscopy.
Klaus Mergener, MD, MBA, FASGE, FACG, AGAF, FACP, FACPE
Affiliate Professor of Medicine, University of Washington, Seattle, WA
A cost-effective, sterile, single-use endoscopic portfolio for the GI space will instantly change the entire practice of gastroenterology. All the concerns with reprocessing and potential cross-contamination would be eliminated. When utilization begins, sterile, single-use endoscopes will represent a classic example of the term “disruptive technology” as applied to endoscopy.
Bergein (Gene) F Overholt, MD,
Past President ASGE, Co-Founder of Gastrointestinal Associates, Knoxville, US
Want to know more?
Do you want to now more about the portfolio of single-use products in Ambu?
Fill in your contact information in the form and we will contact you.
References
J. Kovaleva et al. 2013; Review “Transmission of Infection by Flexible Gastrointestinal endoscopy and Bronchoscopy” Clinical Microbiology Revives April 2013 vol. 26 no. 2 231-254
ECRI institute https://www.ecri.org
CDC Guideline 2008.Disinfection and Sterilization in Healthcare Facilities
FDA Reprocessing of Reusable Medical Devices: Information for Manufacturers https://www.fda.gov/MedicalDevices/DeviceRegulationandGuidance/ReprocessingofReusableMedicalDevices/
AAMI Releases ‘Must-Have’ Guide for Endoscope Reprocessing http://www.aami.org/newsviews/newsdetail.aspx?ItemNumber=2468
CDC safety alert September 11, 2015 “Immediate Need for Healthcare Facilities to Review Procedures for Cleaning, Disinfecting, and Sterilizing Reusable Medical Devices” https://emergency.cdc.gov/han/han00382.asp
CDC release from the Healthcare Infection Control Practices Advisory Committee (HICPAC) January 25, 2017. “Essential Elements of a Reprocessing Program for Flexible Endoscopes – Recommendations of the HICPAC” https://www.cdc.gov/hicpac/recommendations/flexible-endoscope-reprocessing.html
Cori L. Ofstead et al 2017. “A GLIMPSE AT THE TRUE COST OF REPROCESSING
ENDOSCOPES: RESULTS OF A PILOT PROJECT” In International Journal of Healthcare Central Service Material Management (www.iahcsmm.org)


